Discovering Breakthroughs for
Immune Mediated Inflammatory Diseases
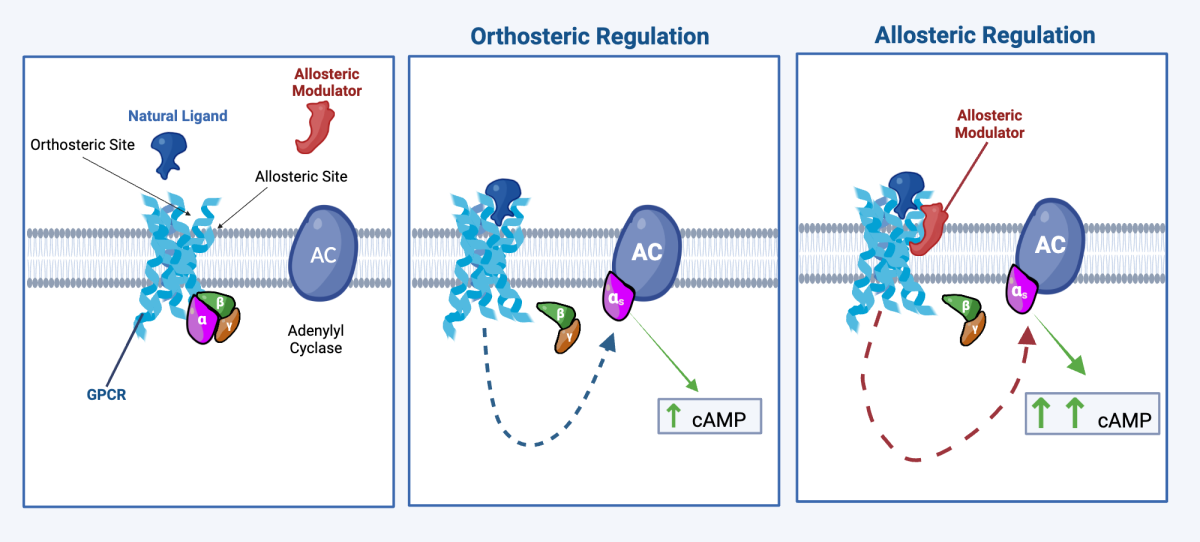
What is GPCR Allosteric Modulation?
Allosteric modulation of GPCRs is the regulation of the GPCR’s activity via the binding of a molecule at a site (the allosteric site) other than the GPCR’s innate ligand binding site (the orthosteric site). Allosteric modulators represent a promising class of small-molecule therapeutic agents, offering distinct advantages over traditional drugs, which typically bind to orthosteric sites. Targeting allosteric sites on GPCRs has the potential to translate into fewer off-target effects and better safety profiles without directly competing with the natural ligand, thereby preserving normal signaling dynamics. Additionally, allosteric sites are generally less conserved across receptor subtypes, offering an opportunity for greater specificity and reduced cross-reactivity, especially in complex receptor families such as GPCRs. These advantages position allosteric small molecules as innovative therapies for inflammatory diseases.

The Role of cAMP in Inflammatory Diseases and Current Approaches:
Cyclic adenosine monophosphate is a critical second messenger in cellular signaling pathways and plays a pivotal role in regulating inflammatory responses. Elevation of cAMP in the presence of inflammation exerts anti-inflammatory effects by suppressing the production of pro-inflammatory mediators (e.g. TNF-α and interleukins) and promoting the release of anti-inflammatory cytokines. Therapeutic strategies aimed at modulating cAMP levels hold great potential to restore immune homeostasis and mitigate inflammatory disease progression.
Challenges with current cAMP related therapies
Challenges with current cAMP related therapies
Phosphodiesterase inhibitors (PDEi) are the primary class of drugs specifically designed to target cAMP levels. These drugs work by blocking the activity of phosphodiesterases (PDEs), the enzymes responsible for the break down of cAMP. By inhibiting PDEs, these drugs prevent cAMP break down and increase intracellular levels of cAMP through accumulation. The primary challenge with PDEi is their narrow therapeutic window and tolerability issues limiting their use.
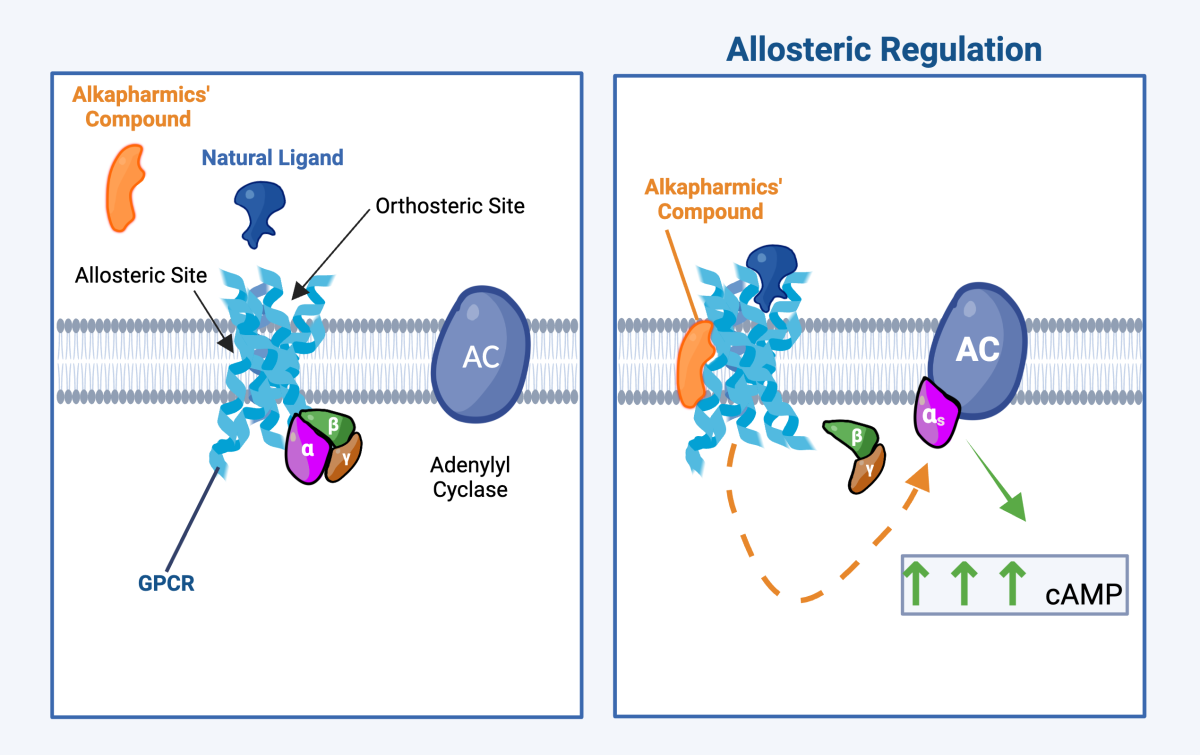
Our Solution: Alkapharmics’ Allosteric GPCR Modulators
Our drugs are small molecules that increase cAMP production selectively, as opposed to inhibition of cAMP breakdown by phosphodiesterase inhibitors. We have demonstrated strong anti-inflammatory effects through numerous in vitro and in vivo inflammatory (innate and adaptive) disease models. The rapid and profound increase in cAMP induced by our drugs results in selective regulation of pro- and anti-inflammatory molecules in innate and adaptive immune cells relevant for a broad range of immune mediated inflammatory diseases. Our drugs can potentially achieve targeted therapeutic effects with favorable therapeutic windows by:
- Producing faster onset of anti-inflammatory effects; resulting in
- Greater early efficacy;
- Potential for more sustained effects;
- Avoidance of target desensitization (often triggered by innate ligands).
All of these anticipated benefits are expected to translate clinically into superior cAMP increases compared to PDEis, and wider therapeutic windows by enhancing natural signaling pathways in a context dependent manner (e.g. presence of inflammation).
By altered GPCR signaling via allosteric modulation and selectively increasing cAMP production in key cell types involved in inflammation, compounds potentially can achieve targeted therapeutic effects with favourable therapeutic window.
We envision becoming the new benchmark in targeted small molecule therapy with multiple potential disease applications, extending beyond the transformative impact of PDEi and JAK inhibitors in managing inflammation and pathological immune responses.
We envision becoming the new benchmark in targeted small molecule therapy with multiple potential disease applications, extending beyond the transformative impact of PDEi and JAK inhibitors in managing inflammation and pathological immune responses.
Entering IND-Enabling Phase to initiate first PoC clinical study in 2026 in dermatology:
Our lead drug is entering an IND-enabling phase, starting with dermatological indications of Atopic Dermatitis and Psoriasis. We expect to enter our first Ph1b clinical trial in the second half of 2026. The initial plan is to deliver the drugs topically to achieve rapid validation of the novel MoA in human inflamed tissue in patients, and to monitor these effects via specific biomarkers of drug activity which are already identified. The team is also advancing its efforts on an oral formulation in parallel to expand to broad inflammatory diseases, beyond dermatology.

